Archived Story
Top News
Latest News
Dexcom launches first over-the-counter continuous glucose monitor.
27 Aug 2024 • 6:26 PM EDT, Ashley Capoot, CNBC
Blood Sugar Tracking Heats Up: Dexcom's Over-the-Counter Sensor Now Available for Sale
27 Aug 2024, Jessica Rendall, CNET
Eli Lilly launches a new, cheaper form of weight loss drug Zepbound for as low as $399 a month
26 Aug 2024 • 8:00 PM EDT, Annika Kim Constantino, CNBC
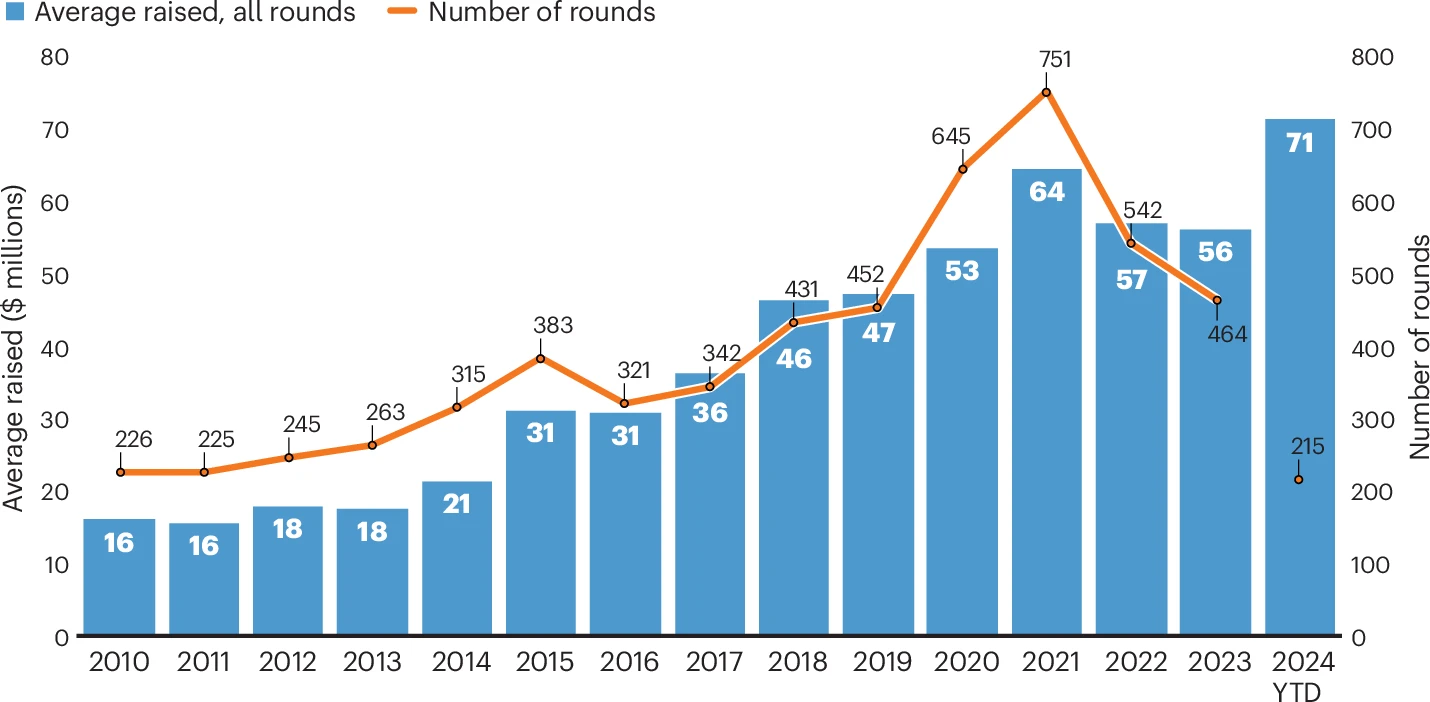
Biotech financing: darkest before the dawn
08 Aug 2024, Melanie Senior, Nature Biotechnology
Abbott Receives U.S. FDA Clearance for Two New Over-the-Counter Continuous Glucose Monitoring Systems
10 Jun 2024, Abbott, Abbott
Gilead Lung Cancer Study Update #Gilead #lungcancer #clinicaltrial GILD
23 Jan 2024 • 11:37 PM EST, YouTube
Is Gilead's 10% Crash — Resulting In A Failed Breakout — An Overreaction?
22 Jan 2024 • 4:08 PM EST, Investor'S Business Daily, Investor's Business Daily
Stock Price, Quote and News
22 Jan 2024 • 10:01 AM EST, CNBC
Gilead Provides Update on Phase 3 EVOKE-01 Study
22 Jan 2024 • 9:53 AM EST, gilead
Humana warns higher medical costs may hit 2024 forecast
18 Jan 2024 • 7:00 PM EST, CNBC